Treating hydrophobic tethers in a temperature-dependent
manner:
There are two ways how hydrophobic tethers are treated during the thermal
unfolding simulation: I) The number of hydrophobic tether constraints
remains constant; II) Hydrophobic tethers are also considered in a
temperature-dependent manner in that the number of hydrophobic tether
constraints increases as the temperatures rises. This follows the idea that
hydrophobic interactions become stronger with increasing temperature. For
more details on how the number of hydrophobic tether constraints is
increased, see "Cutoff for identifying hydrophobic tethers"
below.
If you suppose that hydrophobic interactions contribute significantly to the
stability of your protein, use alternative II); otherwise, alternative I) is
recommended, which is also the default.
Method for placing hydrophobic tethers in the network:
Sets the method according to which hydrophobic tethers are placed between
carbon and sulfur atoms in the network. For each function, all conditions
(listed below) less than or equal to the method value are to be satisfied
for placing a hydrophobic tether. A value of 1 places the most hydrophobic
tethers whereas a value of 3 places the least.
1. Carbon-carbon, carbon-sulfur, or sulfur-sulfur atom pairs satisfy the
cutoff for identifying hydrophobic tethers (see below)
2. Each carbon and/or sulfur in the tether is only bonded to carbon, sulfur
or hydrogen.
3. A given atom is only allowed to have one tether to another residue.
Allowed values: 1, 2 or 3.
Cutoff for identifying hydrophobic tethers (in Angstrom):
Sets the cut-off for identifying hydrophobic tethers (Dcut,hp). This
cutoff defines the maximum distance between vdW radii of two carbon and/or
sulfur atoms between which a hydrophobic tether is formed.
In the case when hydrophobic tethers are not treated in temperature-dependent manner, this cutoff remains constant throughout
the simulation, which means that the number of hydrophobic tether
constraints is not changed during the simulation. In contrast, when hydrophobic
tethers are treated in a temperature-dependent manner, the number of hydrophobic tether
constraints increases during the unfolding simulation by Dcut,hp being
linearly increased from c_start (initial value) to c_stop (terminal value)
for Ecut,hb = 0.0 to Ecut,hb = -6.0 according to the
following equation, which is described here.
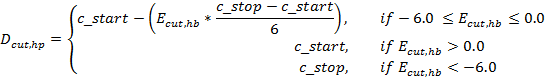
No. of ydrophobic tethers remains constant during simulation:
Between 0.25 and 0.40.
No. of hydrophobic tethers increases with increasing temperature:
Initial value: Between 0.25 and 0.35.
Terminal value: Between 0.35 and 0.45.
(Terminal value must be greater than the initial value)
Energy cutoff for hydrogen bonds (in Kcal/mol):
Sets the energy cut-off (Ecut,hb) for including hydrogen bonds and salt
bridges in the network by defining the range and the step size how hydrogen
bonds and salt bridges will be diluted from the network during the thermal
unfolding simulation. The initial network will have all hydrogen bonds with
an energy Ehb < initial value included. The Ecut,hb is decreased by the step
size until the terminal value is reached. A smaller step size will result in
more steps in the thermal unfolding trajectory.
Initial value: Between -0.1 and -1.0.
Terminal value: Between -5.0 and -10.0.
Step size: Between 0.05 and 0.2.
Number of fuzzy network topologies:
Sets the number of network topologies that are created by fuzzy-constraint
definition when a network ensemble-based approach is requested using a single
input structure.
Allowed values: Between 5 and 500.
Write stability map:
Defines whether or not to calculate a (residue) pair-wise stability map.
The calculation of a stability map is time consuming and hence should
only be requested if needed.